Alberto J. L. Macario, and Everly Conway de Macario
Summary
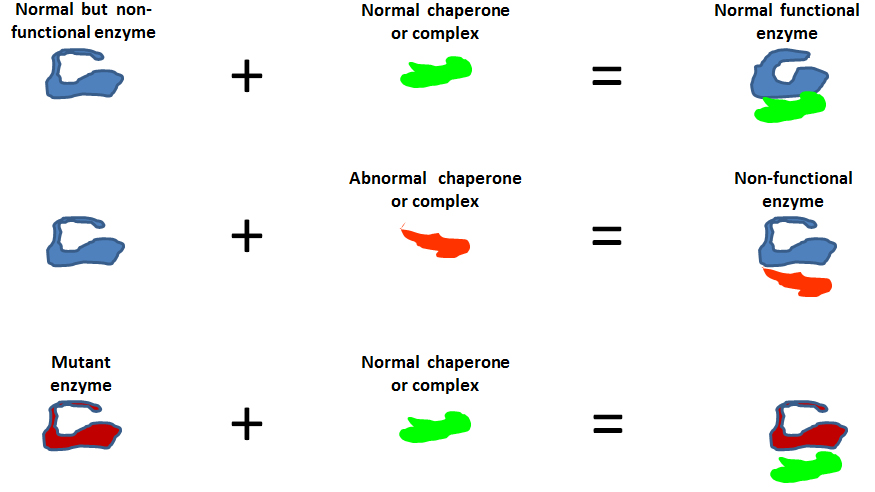
Inherited metabolic syndromes caused by enzymatic deficiencies are typically detected through newborn screening. In most cases, a mutation in the gene encoding the defective enzyme is the pathogenic factor. However there are cases, the frequency of which is currently unknown but it can be assumed to be considerable, in which a defect in the chaperoning system contributes to disease development. The chaperoning system is responsible for the correct folding of enzymes and for maintaining their functional configuration and assembly but when a chaperone is defective the pertinent enzyme is totally or partially inactive. It is now known that in some metabolic syndromes the enzymopathy is caused by chaperone deficiency; it is in fact a genetic chaperonopathy with the gene encoding the enzyme being normal but its product is abnormal due to improper chaperoning. Likewise, cases of autoinflammation caused by mutation of the gene encoding the anti inflammation protein pyrin can be complicated by a concomitant mutation in the gene encoding the ER chaperone TRAP1. This is an example of diseases considered monogenic that can in fact be sometimes digenic: a chaperonopathy acts as a second pathogenic factor, making the clinical-pathological picture very severe. These chaperonopathies can be classified as hidden because they are overshadowed by a clinical-pathological picture characteristic of a known condition typically not linked to a failure of the chaperone system. Physicians and pathologists should be on the alert toward diagnosing chaperonopathies because patients will greatly benefit by proper treatment, including chaperonotherapy.
References
Anikster Y, Haack TB, Vilboux T, Pode-Shakked B, Thöny B, Shen N, Guarani V, Meissner T, Mayatepek E, Trefz FK, Marek-Yagel D, Martinez A, Huttlin EL, Paulo JA, Berutti R, Benoist JF, Imbard A, Dorboz I, Heimer G, Landau Y, Ziv-Strasser L, Malicdan MCV, Gemperle-Britschgi C, Cremer K, Engels H, Meili D, Keller I, BruggmannR, Strom TM, Meitinger T, Mullikin JC, Schwartz G, Ben-Zeev B, Gahl WA, Harper JW, Blau N, Hoffmann GF, Prokisch H, Opladen T, Schiff M.Biallelic mutations in DNAJC12 cause hyperphenylalaninemia, dystonia, and intellectual disability. Am J Hum Genet. 2017 Feb 2;100(2):257-266. doi: 10.1016/j.ajhg.2017.01.002. Epub 2017 Jan 26. PMID:28132689.
Blau N, Martinez A, Hoffmann GF, Thöny B.DNAJC12 deficiency: A new strategy in the diagnosis of hyperphenylalaninemias.Mol Genet Metab. 2018 Jan;123(1):1-5. doi: 10.1016/j.ymgme.2017.11.005. Epub 2017 Nov 20. Review. PMID:29174366.
Feng Y, Liu S, Tang C, Jiang X, Tang F, Li B, Jia X, Chen Q, Liu J, Huang Y.Identification of an inherited pathogenic DNAJC12 variant in a patient with hyperphenylalalinemia.ClinChim Acta. 2019 Mar;490:172-175. doi: 10.1016/j.cca.2018.09.002. Epub 2018 Sep 1. PMID:30179615.
Macario AJL, Conway de Macario E.Sick chaperones, cellular stress, and disease.N Engl J Med 2005; 353:1489-1501.
Macario AJL, Conway de Macario E. Molecular mechanisms in
chaperonopathies: clues to understanding the histopathological abnormalities
and developing novel therapies.
J Pathol.
2019 Oct 3. doi: 10.1002/path.5349. J Pathol. January 2020; 250(1):9-18. PMID:31579936.
Macario AJL, Conway de Macario E, Cappello F. The Chaperonopathies. Diseases with defective molecular chaperones. Springer Dordrecht Heidelberg New York London. (2013).
Macario AJL, Conway de Macario E. Chaperone proteins and chaperonopathies. Stress Physiology, Biochemistry, and Pathology. Handbook of Stress, Volume 3, Chapter 12. Pages 135-152. Edited by: George Fink. Elsevier/Academic Press, 2019.
Standing AS, Hong Y, Paisan-Ruiz C, Omoyinmi E, Medlar A, Stanescu H, Kleta R, Rowcenzio D, Hawkins P, Lachmann H, McDermott MF, Eleftheriou D, Klein N, Brogan PA. TRAP1chaperone protein mutations and autoinflammation. Life Sci Alliance. 2019 Dec 27;3(2). pii: e201900376. doi: 10.26508/lsa.201900376. Print 2020 Feb. PMID:31882397.
The Chaperonopathies Website http://www.chaperones-pathology.organd related Website updates https://www.iemest.eu/life-safety-and-security/images/Doc/ARTICOLI/2016/macario_24/Macario04.pdf;andhttp://journal.frontiersin.org/researchtopic/4348/pathologic-conditions-of-the-human-nervous-and-muscular-systems-associated-with-mutant-chaperones-mo; andhttps://www.frontiersin.org/research-topics/10435/physiology-and-pathophysiology-of-heat-shock-protein-60
Veenma D, Cordeiro D, Sondheimer N, Mercimek-Andrews S. DNAJC12-associated developmental delay, movement disorder, and mild hyperphenylalaninemia identified by whole-exome sequencing re-analysis.Eur J Hum Genet. 2018 Dec;26(12):1867-1870. doi: 10.1038/s41431-018-0237-9. Epub 2018 Aug 23. PMID:30139987.